1.1.실험 목적 ························································
1.2.반응 속도 식·····················································
1.2.1.반응 속도식의 정의······································
1.2.2.반응차수··················································
1.2.3.반응 속도 상수 ··········································
1.3.반응기의 분류····················································
1.4.반응기 설계를 위한 기초식 유도 ······························
1.5.화학반응의 속도론적 해석 ···································
1.5.1.회분식 반응기에 의한 해석 ····························
2.Experiment ····························································
2.1.실험방법 ·························································
2.1.1.실험 기구 및 시약 ······································
2.1.2.실험 방법·················································
3.Results & Discussion ···············································
3.1.Raw data & Calculation ······································
3.1.1.Raw data ···············································
3.1.2.Calculation···············································
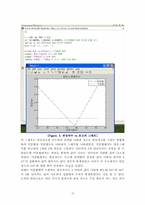
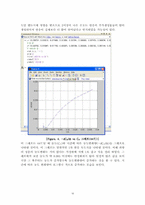
3.2.Matlab ··························································
4.Conclusion ····························································
Reference ···································································
1.1. 실험 목적
회분식 반응기에서 에틸아세테이트의 분해 반응을 용액의 전기 전도도를 실시간으로 측정하여 회분식 반응기의 기초 몰 수지식을 활용하여 반응 속도 상수를 구해 낸다.
농도를 시간에 따라 측정한 후 자료 해석의 미분법 또는 적분법 중의 어느 하나를 이용하여 반응차수와 반응 속도 상수를 구한다.
1.2. 반응 속도식
1.2.1. 반응 속도식의 정의
다음의 균일계의 화학반응을 생각해 보자.
(1.1)
이 반응의 빠르기는 특정성분의 반응계 단위부피, 단위시간당 반응(소실 또는 생성)한 몰 수로 표시되며, [㏖/dm3·s]이다. 반응물질 A에 대해서는 시간적으로 감소하므로 -rA이며, 생성물질 R에 대해서는 rR로 나타내어지며, 이들 사이에 양론 계수를 이용해서
(1.2)
2개 이상의 상이 관계되는 불균일계 반응에서는 반응계의 부피 대신에 계 면적이나 촉매의 질량이 기준으로 사용되며, 기-고 촉매반응속도를 -rA′[㏖/g촉매·s], 기-액 계 반응속도는 -rA〃[㏖/㎡계면적·s]가 채용된다.




















 분야
분야


