Abstract
Introduction
Theory
Experimental
Gold growth procedure by Frens method
Preparation of Au Sols in toluene
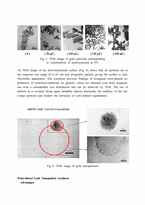
Results and Discussion
Water-Based Gold Nanoparticle Synthesis
Advantages
Disadvantages
Organic-Solution-Based Synthesis of Gold Nanoparticles
Advantages
Disadvantages
Conclusion
References
In general, preparation methods for nanoparticles make use of capping agents, such as surfactants, polymers or biomaterial, in order to confine the growth. Occasionally, the selective or preferential adsorption of capping agents onto particular crystallograp- hic facets during the growth stage permits diverse shapes to be produced, for example triangles, hexagons, disks, rods and multipods. Here, the capping agent functions by inhibiting the growth of a particular crystallographic direction. Among the shapes whi- ch can be obtained, rod-like gold nanostructures have been observer or synthesized using various chemical approaches. In this paper, we report the successful aspect ratio control of gold nanorods by the seed-mediated method, along with an improvement in the yield. Two major parameters were controlled to optimize the growth properties of the gold nanorods, namely their kinetics and anisotropic growth. In the case of the first parameter, we observed that slowing the growth of the Au nanorods increases their aspect ratio. In other words, anisotropic growth can be facilitated at low temper- ature. Recently, it has been found that a range of ordered superstructures of particles can be obtained by similar self-assembly method. Murray and co-workers have develo- ped elegant ways of preparing functionalized and poly-hetero functionalized particles which are of interest for innovative catalytic applications.
The solution-phase reaction of Au nanoparticles with organic dithiols leading to the self-assembly of cross-linked nanonetworked with nanometallic electronic conductivity has been reported in a previous communication. Bard and co-workers have extended this method for the fabrication of self- assembled spherical ultramicroelectrodes by confining the self-assembly process to the tip end of a quartz micropipet.
The present paper focuses on the unusual optical and electronic properties of multila- yer thin films formed by stepwise self-assemblyof ~6 nm Au particles and organic di- thiol on mercaptosilane-functionalized glass substrates. The thin films produced have been studied by UV/Vis spectroscopy, ellipsometry, scanning tunneling microscopy, and temperature-dependent conductivity measurements. The results show that the Au parti- cles are well-protected by the dithiol linker molecules and do not fuse into larger un- ited. The thin film material obtained clearly exhibits nonmetallic properties(i.e., electro -hopping conductivity and optical constants very different from those of metals).
Experimental
Preparation of gold seed solution
2. Cao, Y.W., Jin, R., Mirkin, C.A.: Chem. Soc. 123, 7961 (2001).
3. Jana, N.R., Gearheart, L., Murphy, C.J. J.: Phys. Chem. B105, 4065 (2001)
4. Chen, S., Fan, Z., Carroll, D.L.: J.Phys. Chem. B106, 10777 (2002)
5. Yu, Y.Y., Chang, S.S., Lee, C.L., Wang, C.R.C.: J. Phys. Chem. B 101,6661 (1997)
6. Kink, S., Mohamed, M.B., El-Sayed, M.A.: J.Phys. Chem. B 103, 3073 (1999)
7. JIN, R., Cao, Y., Mirkin, C.A., Kelly, K.L. et al.: Science, 294, 1901 (2001)
8. Obare, S.O., Jana, N.R., Murphy, C.J.: Nano Lett., 1, 601 (2001)
9. Mukherjee, P., Ahmad, A., Mandal, D. et al., Nano Lett., 1, 515 (2001)
10. Nikitenko, S.I., Koltypin,, Y., Masta Y. et al., Chem., 12, 1450 (2002)










 분야
분야


